Scientists Team Up to Unlock DFMO Drug Potential
January 9, 2026
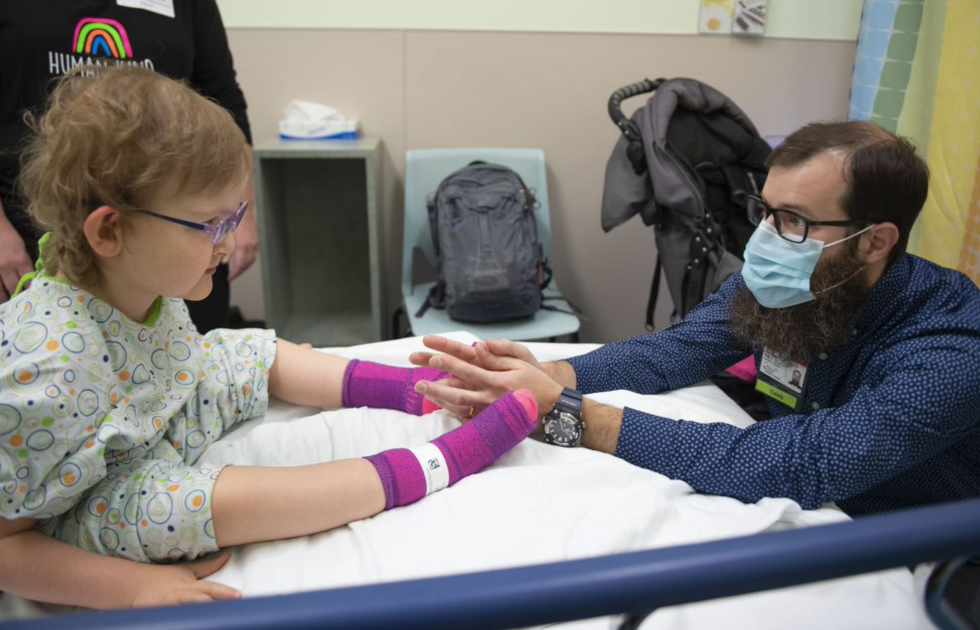
A decades-old drug, long used to treat conditions including a chronic parasitic disease, may soon offer new hope for even more patients with a different life-threatening and ultra-rare disorder, Bachmann-Bupp syndrome (BABS), based on early observations.
The work is made possible through a new partnership between Corewell Health, Michigan State University and Every Cure, a nonprofit biotech organization dedicated to expanding the use of existing medicines to treat other diseases.
 “Beyond helping us build preclinical studies and retrospective analyses, the team at Every Cure has already begun helping us navigate regulatory pathways and compliance on so many levels in the hopes that we can treat more of our BABS patients,” said Caleb Bupp, M.D., pediatric geneticist for Corewell Health Helen DeVos Children’s Hospital in Grand Rapids, Michigan. “They are opening doors that we never would have been able to crack open. It’s a hopeful and exciting time for all of us and more importantly, our patients.”
“Beyond helping us build preclinical studies and retrospective analyses, the team at Every Cure has already begun helping us navigate regulatory pathways and compliance on so many levels in the hopes that we can treat more of our BABS patients,” said Caleb Bupp, M.D., pediatric geneticist for Corewell Health Helen DeVos Children’s Hospital in Grand Rapids, Michigan. “They are opening doors that we never would have been able to crack open. It’s a hopeful and exciting time for all of us and more importantly, our patients.”
The drug difluoromethylornithine, also known as DFMO or eflornithine, has historically been used to treat West African sleeping sickness, a chronic disease caused by the tsetse fly, but it can also help reduce female facial hair growth and prevent the recurrence of neuroblastoma.
Yet, Corewell Health physicians and Michigan State University College of Human Medicine scientists discovered that the drug may also combat the life-threatening neurodevelopmental disorder Bachmann-Bupp syndrome and have already treated some patients under an FDA-approved, single-patient investigational protocol.
BABS is caused by gain-of-function mutations in the ornithine decarboxylase, or ODC1 gene, causing severe neurodevelopmental delay, poor muscle tone and hair loss. DFMO is a well-characterized inhibitor of the ODC protein and can slow down enzyme activity produced by the mutant gene. This has led to the improvement of many symptoms experienced in a limited number of patients.
 “I’ve studied DFMO and its effect on the ODC1 gene for three decades, including its clinical use in pediatric neuroblastoma,” said MSU pediatrics professor André Bachmann, Ph.D., who along with Dr. Bupp collaborated to be the first to identify BABS in a patient. “It was a chance encounter with Dr. Bupp that we connected and were able to use DFMO on a patient – and now five others – with promising early results.
“I’ve studied DFMO and its effect on the ODC1 gene for three decades, including its clinical use in pediatric neuroblastoma,” said MSU pediatrics professor André Bachmann, Ph.D., who along with Dr. Bupp collaborated to be the first to identify BABS in a patient. “It was a chance encounter with Dr. Bupp that we connected and were able to use DFMO on a patient – and now five others – with promising early results.
Since the research team’s discovery, regulatory hurdles, limited awareness and the complexity of launching a clinical trial have slowed treatment progress for this newly described condition, which to date has been reported in only about 20 cases worldwide.
The Food and Drug Administration (FDA) has urged Drs. Bupp and Bachmann to move forward with a clinical trial. However, the team continues to face challenges in raising awareness of DFMO’s potential, developing a robust study design and establishing appropriate clinical endpoints.
“For the past year, we’ve been at a standstill as far as moving our DFMO therapy forward,” Dr. Bupp said.
That is where Every Cure can help. The nonprofit will help reduce many of these barriers and support systems that help BABS patients receive appropriate evaluation and care as potential DFMO therapies are developed.
“Our role is to help bridge this gap by strengthening the evidence behind BABS and DFMO through preclinical studies, increasing awareness among physicians and rare disease organizations, and ensuring that no child goes undiagnosed or untreated,” said David Fajgenbaum, M.D., co-founder and Every Cure president.
Work has already begun between the three organizations to help build on the years of foundational research that Drs. Bachmann, Bupp and Corewell Health colleague Surender Rajasekaran, M.D., have compiled related to DFMO and BABS. The team hopes to have a preclinical study up and running sometime next year.

